Clinical trials are essential to advancing medical science, providing the foundation for new therapies and drugs. However, the success of these trials heavily relies on the integrity and efficacy of trial materials, such as investigational drugs, biological samples, and vaccines. Ensuring these sensitive materials are maintained at the correct temperatures throughout storage and transit is critical. The cold chain is vital in protecting these materials and supporting successful trial outcomes.
The Severity of Temperature Control in Clinical Trials
 Temperature control is paramount in clinical trials due to the high sensitivity of trial materials. Biological samples and investigational drugs are prone to degradation if exposed to temperatures outside their required ranges. Even minor temperature deviations can compromise the safety and efficacy of these materials, leading to inaccurate trial results, regulatory non-compliance, or product spoilage.
Temperature control is paramount in clinical trials due to the high sensitivity of trial materials. Biological samples and investigational drugs are prone to degradation if exposed to temperatures outside their required ranges. Even minor temperature deviations can compromise the safety and efficacy of these materials, leading to inaccurate trial results, regulatory non-compliance, or product spoilage.
Cold chain logistics ensure that clinical trial materials remain stable, from manufacturing to patient administration. By maintaining strict temperature controls, the cold chain preserves the integrity of these materials, ultimately safeguarding the accuracy and reliability of trial results.
Use of Specialized Packaging for Clinical Trial Materials
 Nordic Cold Chain Solutions provides a comprehensive range of specialized packaging products tailored for clinical trials. These products ensure the safety and efficacy of sensitive materials throughout the supply chain and are designed to maintain precise temperature ranges over extended periods. Each packaging solution is pre-qualified to guarantee consistent performance and compliance with the rigorous demands of clinical trials, supporting reliable and accurate trial outcomes.
Nordic Cold Chain Solutions provides a comprehensive range of specialized packaging products tailored for clinical trials. These products ensure the safety and efficacy of sensitive materials throughout the supply chain and are designed to maintain precise temperature ranges over extended periods. Each packaging solution is pre-qualified to guarantee consistent performance and compliance with the rigorous demands of clinical trials, supporting reliable and accurate trial outcomes.
One key component of Nordic’s specialized packaging is gel packs, which are vital for maintaining the stability of temperature-sensitive clinical trial materials. Gel packs are highly efficient refrigerants that absorb and release heat as they transition between solid and liquid states, making them ideal for maintaining controlled temperatures during transit.
Nordic Gel Packs are available in various configurations and temperature ranges, including frozen, refrigerated, and ambient options, allowing them to be precisely matched to the specific needs of clinical trial shipments. These packs offer reliable temperature control, particularly during extended transportation times, when maintaining the stability of sensitive materials is critical.
Key Benefits of Nordic Gel Packs in Clinical Trials:
- Consistent Temperature Control: Gel packs provide a reliable source of cooling, helping to maintain the required temperature range for investigational drugs, biological samples, and other temperature-sensitive materials. This consistency is essential in preventing temperature excursions that could compromise the trial’s integrity.
- Versatility and Customization: Nordic offers a wide variety of gel packs, enabling customization according to the exact specifications of each shipment. This flexibility ensures that each clinical trial shipment is protected against temperature fluctuations that could impact product efficacy.
- Extended Cooling Duration: Designed for long-lasting performance, our gel packs can maintain stable temperatures over extended periods, making them especially valuable when transit times are prolonged or unpredictable. This helps to safeguard trial materials against unexpected delays.
- Compliance with Regulatory Standards: All gel packs are designed to meet industry standards for temperature-sensitive shipping, ensuring clinical trial managers can confidently comply with stringent regulatory requirements.
- Eco-Friendly Options: Our gel packs are also available in sustainable formulations, reducing the environmental impact of cold chain logistics without compromising performance. This commitment to sustainability aligns with the growing emphasis on eco-friendly practices in clinical trials and pharmaceutical logistics.
Real-Time Monitoring and Data Collection
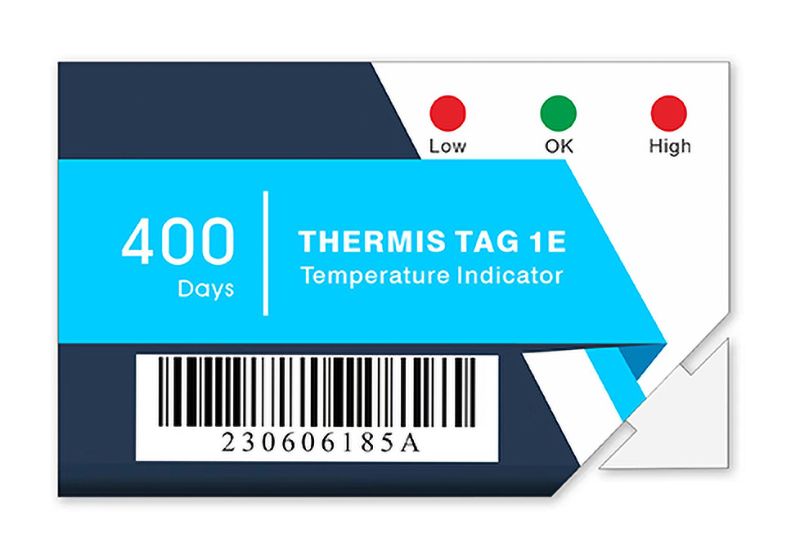 Accountability and transparency are critical in clinical trial supply chains, where even minor temperature deviations can compromise trial results. Nordic’s real-time tracking solutions, including the Thermis® Tag 1E Single-Use LED Temperature Indicator, empower clinical trial managers to closely monitor trial materials’ temperature, location, and humidity throughout the entire transit process. The Thermis® Tag 1E provides immediate visual feedback with its LED indicator, alerting handlers to temperature excursions and enabling prompt corrective actions to maintain material integrity.
Accountability and transparency are critical in clinical trial supply chains, where even minor temperature deviations can compromise trial results. Nordic’s real-time tracking solutions, including the Thermis® Tag 1E Single-Use LED Temperature Indicator, empower clinical trial managers to closely monitor trial materials’ temperature, location, and humidity throughout the entire transit process. The Thermis® Tag 1E provides immediate visual feedback with its LED indicator, alerting handlers to temperature excursions and enabling prompt corrective actions to maintain material integrity.
Continuous monitoring ensures that trial materials remain within the required temperature range, reducing compromised efficacy risk. Real-time data collection also ensures regulatory compliance and strengthens the reliability of clinical trials by providing a comprehensive record of environmental conditions that could impact trial outcomes. By leveraging advanced monitoring technologies, clinical trial managers can effectively mitigate risks, protect the quality of their materials, and support successful trial results.
Ensuring Success in Clinical Trials Through Advanced Cold Chain Solutions
 The integrity of clinical trial materials is paramount to the success of any trial. By leveraging advanced cold chain technologies, clinical trial managers can ensure the safety and efficacy of their products, supporting accurate and reliable trial outcomes.
The integrity of clinical trial materials is paramount to the success of any trial. By leveraging advanced cold chain technologies, clinical trial managers can ensure the safety and efficacy of their products, supporting accurate and reliable trial outcomes.
Nordic Cold Chain Solutions is committed to protecting the integrity of clinical trial materials through innovative cold chain logistics solutions.







