
The pharmaceutical industry is experiencing unprecedented growth in the GLP-1 receptor agonist market, with analysts projecting the global market to reach $100 billion by 2030. As these medications become increasingly central to diabetes management and weight loss treatment protocols, specialty pharmacies face expanding responsibilities in maintaining precise temperature control throughout the distribution chain. With more patients receiving these temperature-sensitive medications at home, ensuring cold chain integrity from pharmacy to patient has become a defining challenge for the industry.
These breakthrough medications are fundamentally changing our approach to diabetes management and weight loss therapy, creating unprecedented opportunities—and challenges—for specialty pharmacies. As demand surges and patient populations expand, maintaining impeccable cold chain integrity has become more crucial than ever before.
Critical Business Impacts:
The regulatory landscape for temperature-sensitive medications continues to evolve, with standards becoming increasingly stringent. USP <1079> provides comprehensive guidelines for storage and transportation, but compliance requires more than simply following a checklist. Successful cold chain management demands a thorough understanding of how these requirements translate into operational excellence.
Critical Compliance Elements:
At the heart of effective GLP-1 distribution is a scientifically-validated cold chain system.
Implementation Considerations:
Consider factors such as their quality management systems, temperature monitoring solutions, and their ability to customize packaging configurations for your specific requirements. A strong cold chain partner will understand regional temperature challenges, provide robust documentation systems that support regulatory compliance, and offer solutions that integrate seamlessly with your chosen shipping methods. They should also demonstrate a commitment to innovation and continuous improvement, staying ahead of industry trends and emerging technologies in cold chain packaging and temperature monitoring.

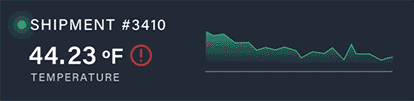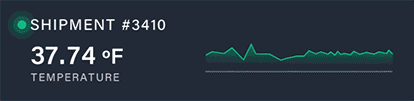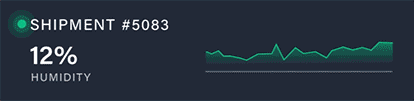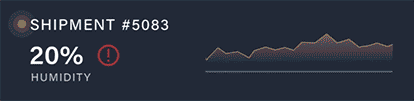
Discover how a high-volume pharmacy optimized its operations, tackled surging demand for GLP-1 medications, and elevated patient care with innovative solutions from Nordic.
Download the Case Study
